Technology
The ENABLR technology platform provides an off-the-shelf solution ideally suited to boost T-cell based therapies.
Immunotherapy is a revolutionary advance in the treatment of cancer, in which we make use of the immune system in order to fight cancer. The immune system can be boosted or altered in such a way that it can find and attack cancer cells.
Known as cellular immunotherapy, this treatment makes use of the cells of the immune system to eliminate cancerous cells.
Some of these approaches involve directly isolating our own immune cells and simply expanding their numbers, whereas others involve genetically engineering our immune cells (via gene therapy) to enhance their cancer-fighting capabilities.
Our immune system is capable of recognizing and eliminating cells that have become infected or damaged as well as those that have become cancerous. In the case of cancer, immune cells known as killer T cells are particularly powerful against cancer, due to their ability to bind to markers known as antigens on the surface of cancer cells. Cellular immunotherapies take advantage of this natural ability and can be deployed in different ways:
Tumor-Infiltrating Lymphocyte (TIL) Therapy
Engineered T Cell Receptor (TCR) Therapy
Chimeric Antigen Receptor (CAR) T Cell Therapy
Natural Killer (NK) Cell Therapy
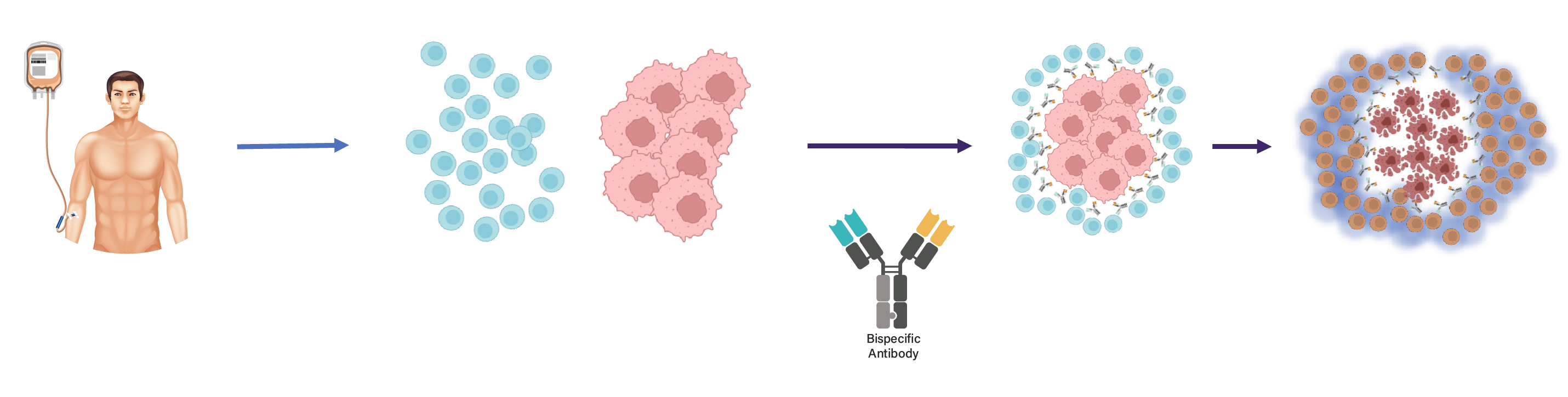
T-BsAb therapy is a form of immunotherapy that enables soldier cells of the immune system to recognize and kill tumour cells, by simultaneous binding to a tumour-associated antigen (TAA) expressed on tumour cells and to CD3 on a T cell (CD3xTAA) . Crosslinking of these two cell types by T-BsAbs allows T-cell activation to kill the tumour cells.
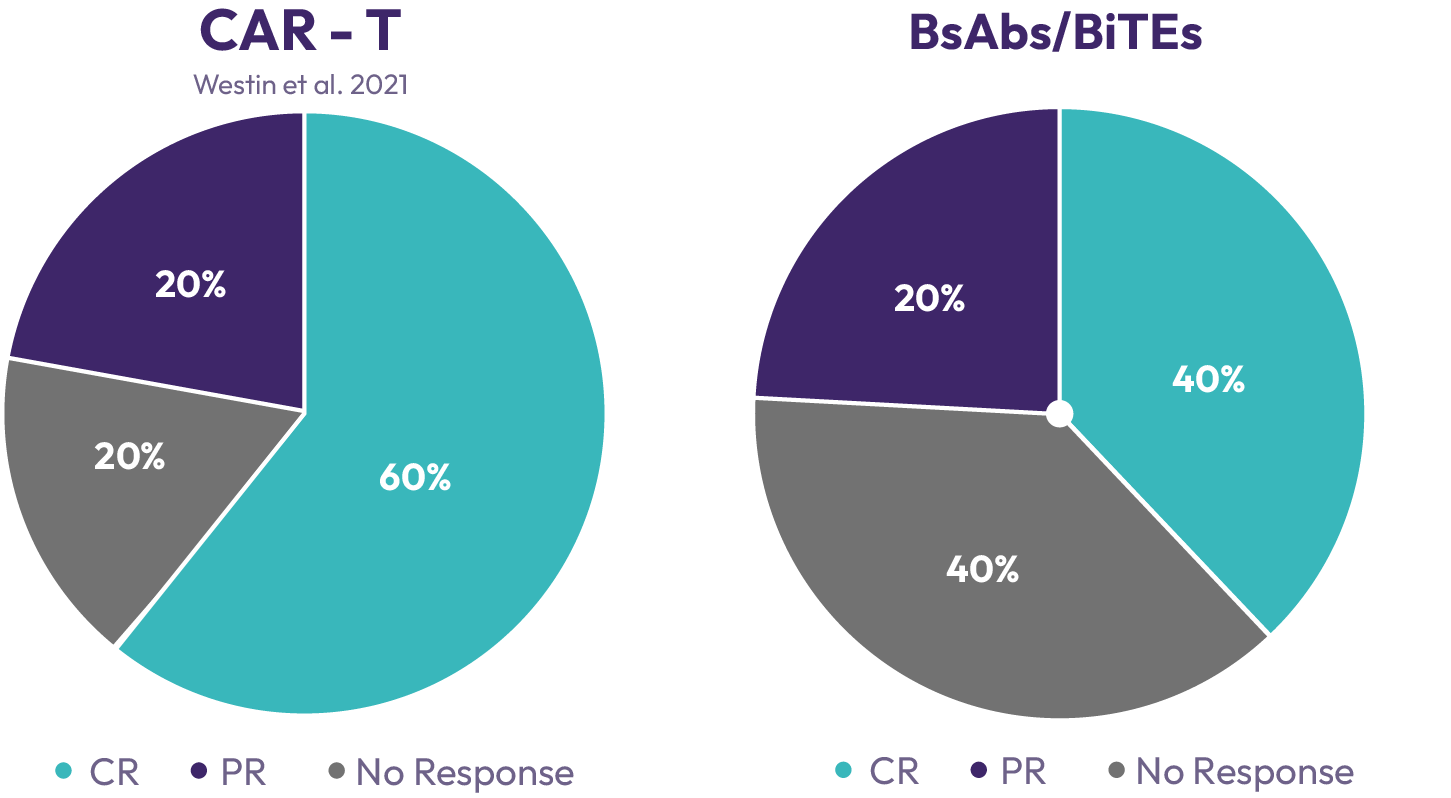
A number of bispecific T-cell engaging antibodies have recently been approved. While their off-the-shelf availability is a distinct advantage over autologous CAR-T cells, they have a higher rate of primary treatment failure and response duration is limited due to conversion of tumour infiltrating lymphocytes to an exhausted phenotype and antigen loss on tumour cells.
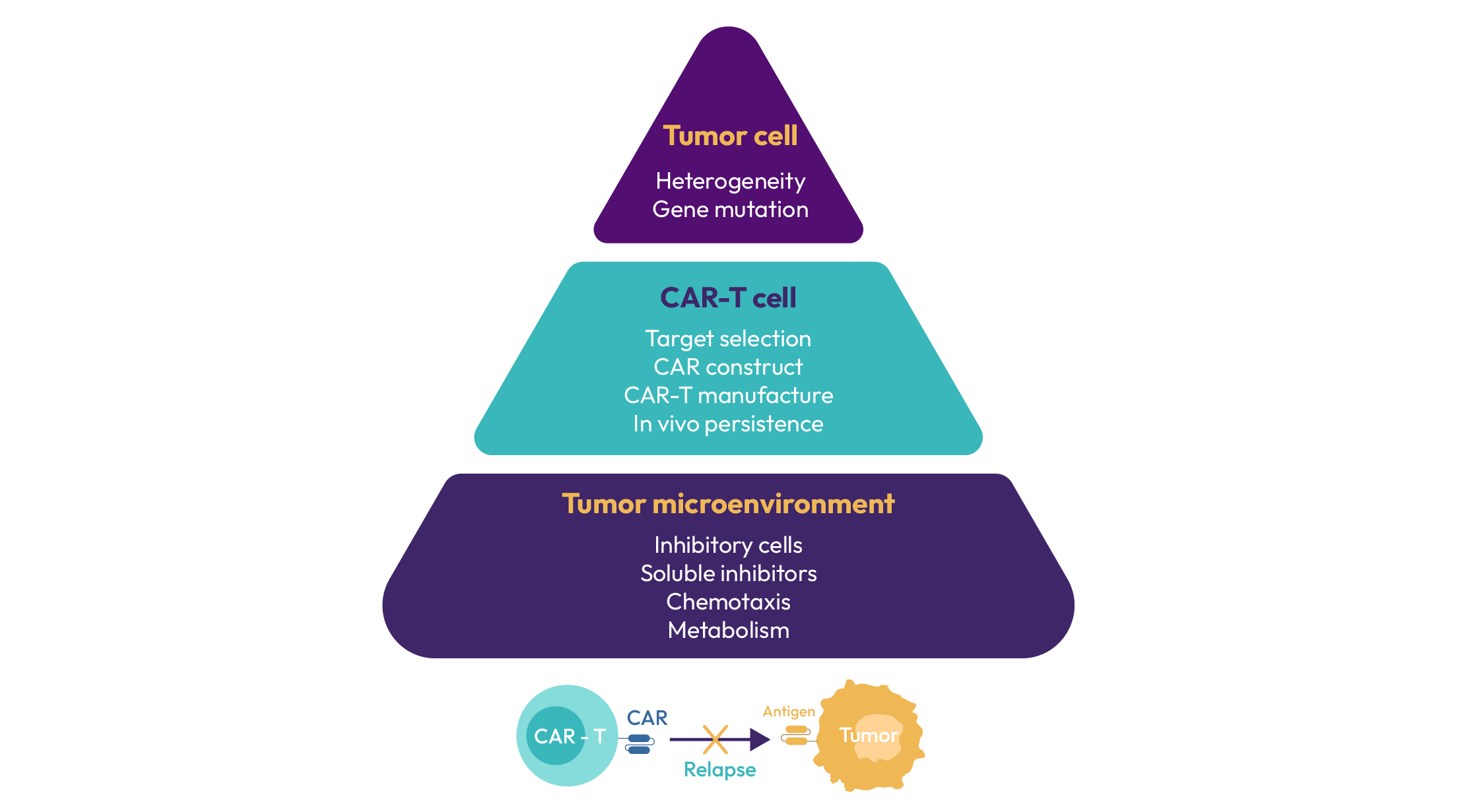
However, there continues to be a high medical need because of multiple deficiencies of these treatment approaches, which limits patient access and therapeutic efficacy.Treatment of solid tumours has met with very limited successwith both approaches. On target off tumour toxicity, and limited capability of the patient’s T-cell to cope with a hostile tumour microenvironment are major contributors to this failure. Moreover, long-term T-cell stimulation leads to an exhausted phenotype with loss of cytotoxic capabilities. As a consequence, patients relapse and are running out of treatment options.

ENABLR serves the most vulnerable patients by our unique offering of safe, more durable and more potent T cell therapy ENABLR (ENineered Antigen Blinded Receptor) T-cell platform are allogenic T-cells engineered to lose antigen recognition by removing the variable domains in the α and β chains of the T-cell receptor; however they retain the CD3 dependent downstream activation machinery.
ENABLR cells therefore are potently cytotoxic to tumour cells when given in conjunction with T-cell engaging bispecific antibodies (TCE) . Due to the lack of antigen recognition GvHD is reliable prevented; the cells can therefore be universally applied.
The ENABLR platform is inherently safe, as the cells are immunologically silent without addition of a bi-specific antibody. Safety is further increased by a suicide switch, which can be activated in case of uncontrolled proliferation. As the technology is not limited by the kind of tumour associated antigen (TAA), ENABLR cells can be also combined with multiple TCEs, and will be activated by them simultaneously.
ENABLR is therefore a first in class technology which combines the benefits of adaptive T-cell transfer with CD3-based T-cell activation using bi-specific antibodies. The technology relieves the dependence of TCEs on the pre-existing landscape of patient T-cells, which in many cases have an abundance of exhausted phenotype due to high tumour burden and persistent activation by tumour antigen. As the cells are derived from healthy donors, they present a consistent and highly activatable population of tumour naive T-cells.

T-cells expressing ENABLR are manufactured using a simple engineering technique, in which healthy T-cells are isolated from healthy donors, genetically engineered using CRISPR technology and viral vectors, and finally purified through selection.